| Whisker refers to a fiber material with a certain aspect ratio grown in the form of a single crystal, and its diameter is about 1-100mm, the length is about 10-1000mm, and the aspect ratio reaches 5-1000 or even higher. It does not contain the defects (grain boundaries, dislocations, holes, etc.) that are usually present in the material, the atoms are highly ordered, and the strength is close to the theoretical value of the complete crystal. The whisker has a certain cross-sectional shape, complete shape, and complete internal structure. It is the solid with the greatest strength known at present, has excellent mechanical properties, and can be used as the reinforcement and modification component of composite materials. However, most of the whisker materials that have been developed at home and abroad, such as silicon carbide whiskers, silicon nitride whiskers, alumina whiskers, etc., are expensive and complex preparation processes, which limit the expansion of their application fields.
Calcium sulfate whiskers, also known as gypsum whiskers, are fibrous single crystals of anhydrous calcium sulfate, white loose needles, with a perfect structure, complete shape, specific cross section, stable size, and its average aspect ratio is generally 30-80. Compared with other inorganic whiskers, calcium sulfate whiskers have high cost performance, abundant raw material resources, simple preparation process, and low price. They are green and environmentally friendly materials. They can be used as reinforcing materials, friction materials, environmentally friendly filter materials, coating paper filling materials, etc. It has broad application prospects and practical value. 
1. Overview of calcium sulfate whiskers
There are three kinds of calcium sulfate whiskers, which are anhydrous calcium sulfate (CaSO4) whisker, calcium sulfate hemihydrate (CaSO4·0.5H2o) whiskers and calcium sulfate dihydrate (CaSO4·2H2O) whiskers. Are fluffy white solid, fibrous or needle-like single crystal morphology. Among them, anhydrous calcium sulfate whisker has the best application prospect in industrial production and can be used as a medium strength enhancer. Its physical properties are: relative molecular mass of 136.14mm, diameter of 0.1-2mm, length of 20-150mm, aspect ratio of 10-100; melting point of 1450 ℃, relative density of 2.96mm, Mohs hardness of 3-4, tensile strength of 2.058Gpa, elastic modulus of 176.4Gpa.
The relative molecular mass of calcium sulfate dihydrate whisker is 172.18, the aspect ratio is generally 15-100, and the heat resistance, hardness and strength are poor. It will be weathered and dehydrated at room temperature, and will continue to dehydrate into amorphous powder at about 110 ℃. Therefore, it has received certain restrictions in industrial applications and is not optimistic.
The relative molecular mass of calcium sulfate hemihydrate whiskers is 145.15, the aspect ratio, heat resistance, hardness and strength are between dihydrate and anhydrous calcium sulfate whiskers, and the calcium sulfate powder is dehydrated to amorphous at about 160 ℃. In industrial applications, it can be converted into anhydrous calcium sulfate whiskers.
The physical and chemical properties of calcium sulfate whiskers are better. Table 1 is a comparison of the properties and prices of calcium sulfate whiskers with other whiskers. As can be seen from the table, although calcium sulfate whiskers are slightly inferior to some excellent whiskers developed in terms of mechanical properties, they have a strong price advantage. It has many raw materials, low cost and relatively simple preparation method. It is a non-metallic green environmental protection material with various properties. It can be used in engineering plastics, papermaking, brake pads and many other fields, and has broad industrial application prospects. Table 1 Comparison of properties and prices of calcium sulfate whiskers and other whiskers 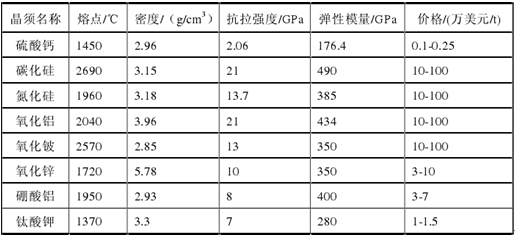
2. Growth mechanism of calcium sulfate whisker
The growth process of anhydrous calcium sulfate whisker can be summarized as the following steps: dissolution → recrystallization → dehydration molding, the specific reaction is as follows:
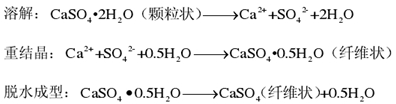
Calcium sulfate whisker is a single crystal, its growth process mainly follows the axial spiral dislocation growth mechanism, in line with the Harlman and Perdoek proposed by the periodic chain theory. The crystal structure of calcium sulfate is [SO42-] Tetrahedron and Ca2 The two-layer axial direction parallel to the (010) plane is formed, which is the main direction of crystal growth. It is precisely because of the difference in growth rate between the axial direction and the side surface that the one-dimensional dislocation extension is completed. Gypsum dissolves and releases crystal water. When the dissolved gypsum reaches supersaturation, recrystallization forms crystal nuclei. The crystal grows on the basis of crystal nuclei, and the axial strong growth rate is the fastest with spiral dislocation proliferation, while the lateral growth is slow or even stagnant. The crystal grows along the axial direction and the dislocation extends continuously, forming the final form of calcium sulfate whisker.
In addition, hemihydrate and dihydrate calcium sulfate whisker is not very stable, easy in the humid air or aqueous solution hydration reaction, resulting in its physical and chemical properties and crystal morphology changes, more research in this area, the results of the surface of the hydration process and mechanism in line with the theory of solution analysis. The hydration reaction affects the storage and use of calcium sulfate whiskers, so calcium sulfate whiskers usually need to be stabilized, such as high temperature calcination, adding stabilizers, surface modification treatment, etc. |









